Glycobiology is central to many important biological and biochemical processes. The interactions of cell surface glycoconjugates with other cells, proteins or pathogenic microorganisms. This process of molecular recognition is the result of interactions with many different binding partners with the carbohydrate structures driving the specificity of the interaction. Fundamental aspects of the function of these glycoconjugates are still not well understood. To determine the functions of these complex molecules we must be able to dissect the biosynthetic process find the control points in these processes and understand the interactions of glycoconjugates with their receptors.
Synthetic Biology and its Application for Glycobiology?
We have been working on improving therapeutic proteins through their glycosylation . To that objective we have been engineering in vivo protein glycosylation through improving landing sites for glycosyltransferases. Our work includes synthetic operons to add biosynthetic pathways glycosylation in our bacterial host, E. coli.
Synthetic biology to make therapeutic proteins – with human glycans!
We have developed a two-plasmid system to produce the target protein and the enzymes needed to glycosylate it. Some targets we are interested include interferons as anti-virals, anti- inflammatories, and colony stimulating factors to help with immune cell generation. We are now on second generation designs.
Cytokines are some of the most potent therapeutic proteins we have.
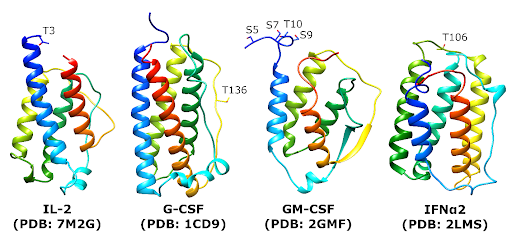
We spend a lot of time thinking about protein structure, and how that relates to where glycosylation occurs, and how we can engineer that to change the properties of cytokines. This includes better stability, lower tendency to aggregate, and longer serum half-life.
Enzymes for Glycobiology?
Our research has been focused on the investigation of the structure and function of the enzymes involved in making various glycoconjugates . The lab studies bacterial enzymes which make carbohydrate structure mimics of the host they infect, as well as the corresponding eukaryotic enzymes. The determination of glycosyltransferase enzyme donor/acceptor specificity as well as contributing to the determination of the 3-dimensional structures has been the major area of research and will continue to be the focus of my collaborative research program. We are also applying these enzymes to the synthesis of bioactive glycoconjugates for various therapeutic applications. Part of this work is the creation of designer enzymes and target proteins for these applications . We are always on the lookout for new enzymes to add to the inventory!
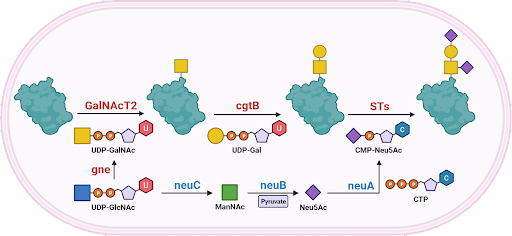
The first GT-52 Sialyltransferase Structure.
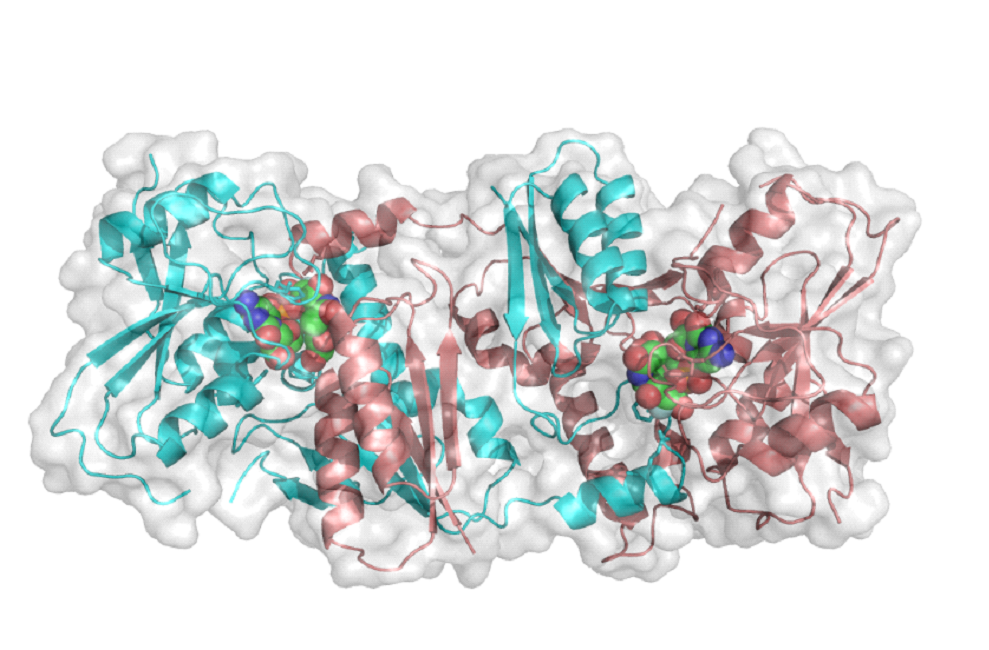
This protein is from the bacterial pathogen Neisseria meningitidis, one of the bacteria that cause meningitis. This enzyme has two identical halves which combine to make the active enzyme. The structure made by this enzyme help protect the bacteria from the immune system.
Bacterial Protein Mannosylation
Protein mannosylation has been observed from bacteria to humans and appears to be a highly conserved protein modification that is essential in eukaryotes. Protein mannosylation in actinobacterial species was originally described over 40 years ago, however we still lack a basic understanding of the importance of this post-translational modification in prokaryotes. Mannosylated proteins have been discovered in many well-known actinobacteria including Mycobacterium tuberculosis, Corynebacterium glutamicum, Cellulomonas fimi, and Streptomyces coelicolor. Functions for only a few of these mannosylated proteins have been discovered, making it difficult to decipher the biological significance of this modification within these bacteria.
The Problem with Protein Mannosylation in Actinobacteria.
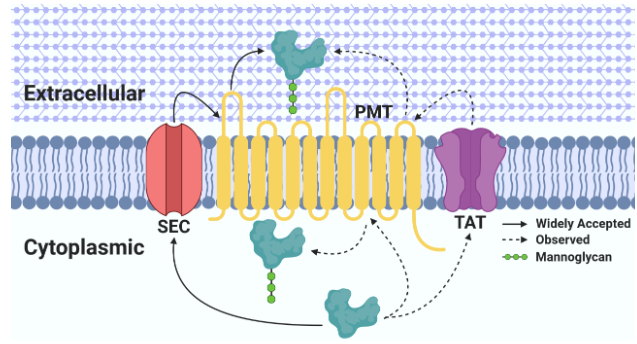
We found that the glycosylation machinery in C. glutamicum was able to use the recombinant C. fimi proteins as substrates and that the glycosylation matched closely that found in the native proteins when expressed in C. fimi. The puzzling thing is how can proteins in the cytoplasm, and secreted by two different pathways all get glycosylated? We are pursuing this observation as a prelude to dissecting the biosynthetic machinery and biological consequences of this protein mannosylation.
Wakarchuk Lab Funding


